The new European Union Medical Device Regulation (EU MDR) has set a high bar for clinical evidence and documentation, making Europe a tightly scrutinized regulatory environment for medical device companies. Industry surveys reveal that a large proportion of technical documentation submissions to Notified Bodies contain substantive gaps, leading to early rejection and extended remediation timelines. These EU MDR compliance pitfalls translate directly into measurable business impact: extended time to market, escalating expenditures, and significant revenue loss from postponed launches.
Restoring market viability under the EU MDR requires focused remediation across a defined set of regulatory and evidence gaps that repeatedly trigger rejections. Syrma Johari MedTech has supported multiple medical device companies through these challenges, towards EU MDR approval. This article examines recurring remediation areas where MedTech teams face delays, demonstrated through real-world cases addressed by our regulatory experts.
1. Clinical Evaluation and Evidence Gaps: The Primary Source of EU MDR Compliance Failures
Industry analyses of deficiency patterns illustrate that gaps in clinical evaluations and clinical evidence are among the most commonly cited non-conformities during EU MDR compliance assessments. Rejection is often triggered when the Clinical Evaluation Report (CER) fails to demonstrate clear linkages between intended use, clinical evidence, and the resulting safety and performance claims:
1A. Case Study – The Stratification Gap: When Broad Claims Lack Specific Evidence
A surgical instrument manufacturer submitted clinical evaluation documentation intended to support use across multiple surgical specialties. However, the documentation failed to define the specific types of procedures for which the device was intended, treating “surgical use” as a single, uniform indication. This reflected a common EU MDR compliance misconception: that one body of clinical evidence can support multiple, clinically distinct applications.
In practice, different surgical procedures present fundamentally different risk profiles, performance expectations, and user interactions. A device used in orthopedic trauma surgery cannot be clinically justified in the same manner as one used in minimally invasive general surgery. Similarly, pediatric use requires separate safety and performance substantiation from adult indications. The submitted CER did not stratify evidence by procedure type or patient population, and the literature search did not adequately cover each claimed use. These gaps weakened the overall clinical strategy and prompted Notified Body rejection.
Remediation Provided by Syrma Johari MedTech
- Syrma Johari MedTech restructured the clinical evaluation to assess each intended procedure as a distinct regulatory assessment, anchored to a clearly defined intended use.
- Targeted literature searches were conducted for each surgical application and relevant patient population.
- State-of-the-art comparators were selected based on the specific procedural context, ensuring that performance benchmarks reflected current clinical practice rather than generalized surgical use.
This stratified, indication-specific approach demonstrated robust substantiation of device performance across all intended applications. It converted a rejected submission into a clinically and regulatorily defensible CER, resulting in prompt EU MDR approval
1B. Case Study – The Equivalence Fallacy: When Public Data Is Not Sufficient for EU MDR Compliance
A manufacturer of a Class IIb implantable device attempted to demonstrate equivalence to a legacy device using only publicly available information. The equivalence rationale relied primarily on apparent similarity in raw materials and intended use, without access to the predicate device’s full technical documentation.
Under the European medical device regulation, equivalence for implantable or higher-risk devices must be demonstrated across clinical, technical and biological characteristics. Public sources did not provide sufficient detail on critical parameters such as material grade, processing controls, manufacturing methods and sterilization validation. Without access to the predicate technical file, these characteristics could not be verified. As a result, the equivalence justification lacked the depth and traceability required under Annex XIV and was rejected by the Notified Body.
Remediation Provided by Syrma Johari MedTech
- Syrma Johari MedTech conducted a structured gap analysis that confirmed equivalence could not be substantiated without proprietary technical file access.
- The regulatory strategy was revised to remove unsupported equivalence claims and replace them with an evidence pathway based on original clinical data.
- Targeted clinical data collection activities were defined within the validated intended use, supported by structured retrospective data review and physician-reported outcomes.
- In parallel, the Risk Management File (RMF) was updated to reflect emerging clinical evidence and to maintain traceability between identified risks, clinical outcomes, and benefit–risk conclusions.
This evidence-driven approach enabled the submission to meet Notified Body expectations without reliance on unverifiable equivalence claims.
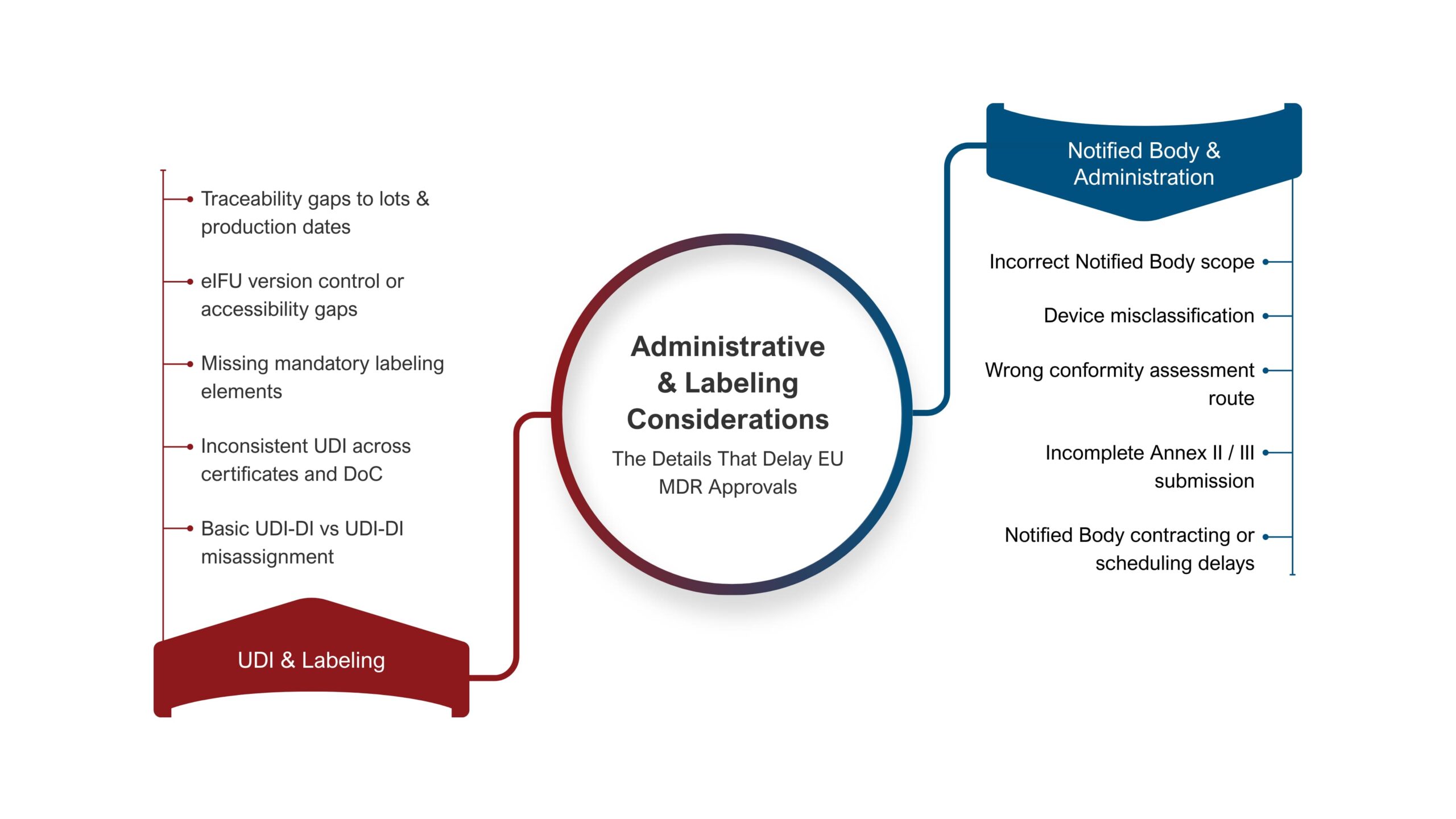
2. Technical Documentation: Structural Shortfalls in EU MDR Compliance
Poor technical documentation remains a frequent reason for the rejection of EU MDR applications. Many MedTech companies continue to rely on outdated ISO standards or mixed approaches that fall short of 2026 state-of-the-art benchmarks. Furthermore, Risk Management Files (RMF) are often not updated with production experience, complaints, vigilance data or emerging clinical knowledge. Under the evolving EU medical device regulation changes, these weaknesses compromise compliance with Annexes II and III and delay conformity assessment.
2A. Case Study – DHF Remediation Triggered by Outdated Standards
A manufacturer of an aesthetic medical device submitted technical documentation assuming that previously approved biocompatibility data remained valid because the product design had not changed. As a result, the Design History File (DHF) was not updated to reflect current biocompatibility expectations required for EU MDR compliance.
During review, the Notified Body identified that the biocompatibility assessment referenced outdated versions of ISO 10993 and did not reflect current state-of-the-art requirements. Under the EU medical device regulation, unchanged design does not exempt a device from reassessment against updated harmonized standards. The absence of a current, risk-based biocompatibility evaluation within the DHF constituted a material non-conformity and triggered remediation.
Remediation Provided by Syrma Johari MedTech
- Syrma Johari MedTech conducted a comprehensive DHF remediation to align the technical documentation with the current ISO 10993 standards.
- The biocompatibility evaluation was re-performed using a risk-based approach appropriate to the device’s nature, duration of contact and anatomical exposure.
- All supporting test reports, rationales and conclusions were updated and cross-referenced within the technical documentation to ensure traceability.
The remediation restored alignment with the latest EU MDR compliance standards and enabled the submission to progress through Notified Body review.
2B. Case Study – Essential Requirements Mistaken for GSPR Compliance
A medical device manufacturer submitted technical documentation supported by an Essential Requirements checklist carried forward from the Medical Device Directive (MDD), assuming it was equivalent to the General Safety and Performance Requirements (GSPR) checklist required under EU MDR. This is a common misinterpretation of MDR requirements, given the recent EU medical device regulation changes.
The EU MDR GSPR framework introduces additional and expanded requirements that go beyond the former Essential Requirements. In this case, the manufacturer had not identified or tested against several MDR-specific GSPR clauses. As a result, the checklist lacked complete coverage and supporting evidence, and multiple requirements were marked as compliant without corresponding verification. The Notified Body identified this as a structural deficiency in the technical documentation and requested remediation.
Remediation Provided by Syrma Johari MedTech
- Syrma Johari MedTech performed a detailed GSPR gap assessment to distinguish legacy Essential Requirements coverage from MDR-specific obligations.
- A complete MDR GSPR checklist was developed, with each applicable requirement mapped to objective evidence.
- Where additional testing or verification was required to meet MDR-specific GSPR clauses, the necessary activities were defined and incorporated into the technical documentation.
- Cross-references were established between the GSPR checklist, risk management documentation, test reports, and clinical evidence to ensure traceability.
The remediation replaced an outdated compliance assumption with a complete, MDR-aligned GSPR framework, allowing the submission to progress through Notified Body review.
3. Post-Market Surveillance: Lack of Proactive Monitoring Drives EU MDR Non-Compliance
The EU medical device regulation requires structured post-market surveillance (PMS) and PMCF (where applicable), particularly for higher-risk devices. Generic PMS plans that lack defined data sources, collection methods, or analysis pathways remain a frequent cause of rejection. Notified Bodies clearly distinguish between active surveillance and PMCF programs and documentation assembled solely to satisfy checklist requirements.
3A. Case Study – Generic PMCF Plans Fall Short of Notified Body Expectations
A manufacturer of an implantable medical device submitted a PMS plan that was largely generic and lacked defined data sources, collection methods and analytical processes. The plan relied on high-level complaint trending and benchmarking, without demonstrating how device-specific clinical performance and safety data would be actively generated or evaluated.
Given the implantable nature of the device, the Notified Body expected a structured PMCF approach for EU MDR compliance. However, the submission omitted the PMCF entirely. The absence of proactive real-world evidence collection was identified as a material deficiency. As a result, the post-market strategy was deemed insufficient under EU MDR expectations and returned for remediation.
Remediation Provided by Syrma Johari MedTech
- Syrma Johari MedTech supported the development of a device-specific PMS framework with clearly defined data sources, analysis methods and review intervals appropriate for an implantable device.
- A focused PMCF plan was established to generate real-world clinical evidence within the approved intended use, including retrospective data from hospital records and electronic health records. This was supported by direct physician input through Syrma Johari MedTech’s established clinical networks in the United States and the United Kingdom.
- PMS and PMCF outputs were integrated into the CER and RMF, restoring traceability across post-market evidence.
This approach replaced a generic post-market plan with an operational surveillance framework that met Notified Body expectations for high-risk devices.
Summary: Mapping Major EU MDR Compliance Failures Across the Regulatory Framework
| EU MDR Compliance Area | Risk Expression |
| Clinical Evaluation (Annex XIV) | Intended use ambiguity, weak stratification, unsupported equivalence |
| Technical Documentation (Annex II) | Outdated standards, GSPR misalignment, incomplete DHF |
| Risk Management (Annex I) | Static RMF, outdated ISO 14971 approach, weak benefit–risk justification |
| Post-Market Surveillance (Annex III) | Generic PMS, missing PMCF, no feedback into CER/RMF |
Establishing EU MDR Compliance on Proven Regulatory Foundations
EU MDR compliance failures often follow recurring patterns. They commonly stem from predictable weaknesses in clinical strategy, technical documentation structure, risk management maturity and post-market evidence generation. The cases above demonstrate that preventing rejections requires precise intended use definitions, current state-of-the-art evidence, integrated risk management, and operational PMS and PMCF systems.
Syrma Johari MedTech has consistently demonstrated the ability to rectify these failures with the technical precision and regulatory discipline expected by Notified Bodies. We have a dedicated, multidisciplinary QARA team with deep expertise in Notified Body reviews. Our experience spans the entire EU MDR compliance framework across the product lifecycle, enabling MedTech companies to scale without disruption to their regulatory compliance.
EU MDR remediation drives up costs and delays market access. Intervene early!
Partner with us for EU MDR compliance that meets Notified Body expectations.

