Medical Device Proof of Concept, Prototype, and MVP (Minimum Viable Product): Same or Different?
Medical Device Proof of Concept, Medical Device Prototype, and MVP (Minimum Viable Product) are often used Interchangeably. This happens in...
Happy Mother’s Day 2022: Nisha Johari on being Mompreneur!
Evolving from a daughter to a grandmother! I had an enriched & cheerful childhood. As a daughter my parents triggered key strengths in...
5T formula for Quality Medical Device Manufacturing
Medical Device Manufacturing in India is undergoing a massive transformation. With digitalization penetrating multiple practices from Medical...
These measures will help Medical Device Manufacturers remain profitable during unprecedented Oil & Gas prices hike!
India is emerging as a potential Medical Device outsourcing hub due to favorable investment schemes, government support and a quality talent...
8 Medical Device Design Flaws and How to fix them
Medical Device Design failure shows its impact both ways for the user and the manufacturer. The overhead cost due to recalls and extra...
Aesthetic Medical Device Technologies and its Manufacturing
The medical aesthetics market is valued at USD 15.9 billion by 2025. With the majority population becoming aware and concerned about their...
Why fragmenting your Medical device manufacturing at different locations is a bad idea?
Every Medical Device Developer wants minimal costs, high quality, and first-mover advantage. But, getting all at once under a single roof is...
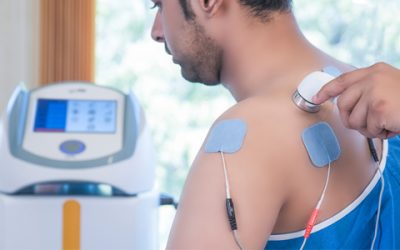
6 Applications of Electrotherapy in Healthcare
Electrotherapy has been used as a pain alleviating treatment for a very long time. To date, its complete potential remains unexplored....
We are expanding our horizons for better healthcare
We are extremely glad to announce that we are expanding to Medical Devices Park, Jodhpur. With this expansion, Syrma Johari MedTech...
Understanding Human Factors for Medical Devices
An intuitive interface and safety are the top priorities for today's Medical device designers. Functionality and aesthetics both play a...
SaMD, Software as a Medical Device and its challenges in regulatory
What is SaMD? As per FDA, the term Software as Medical Device (SaMD) is Defined as "software intended to be used for one or more medical...
Health Canada Approval Process for Medical Devices: Step-by-Step Guide
The Canadian market has stringent regulations concerning Class I, II, Class III, Class IV Medical Devices. If you are someone looking to...
Do’s & Don’ts for a Medical Device Startup
There has been a gradual shift in the Startup ecosystem in India. Three major influences controlling the economy dynamics, Government,...
Labor Pain Management by TENS : Why or Why not?
Labor pain is one of the most severe kinds of pain a women experiences in their lifetime. There have been many instances, where doctors have...
What is Medical Device Contract Manufacturing and How it helps to OEM?
What is Medical Device Contract Manufacturing? Medical Device Contract Manufacturing is a process where a MedTech company that owns the idea (sole...