Entering the European Market under the EU MDR is no longer about meeting isolation requirements. However, it is considered one of the most tedious tasks. This is due to the lack of technical understanding, and more objective considerations. It is at this time we would like to decipher for you, where you stand and how to take steps further.
We have seen repeatedly that it is not the manufacturers who believe they are already compliant that move forward with confidence but those who can demonstrate readiness across quality, clinical, and regulatory dimensions in a coherent and defensible way. We have designed a gap assessment worksheet that suffices as a Single Stop Window to assess your MDR Current Readiness
The checklist systematically maps each applicable MDR requirement to corresponding sections of the technical documentation, ensuring clear traceability, consistency, and completeness across the entire medical device lifecycle. It covers key elements including medical device description, design and manufacturing information, risk management, design verification and validation activities, clinical evaluation, and post-market surveillance.
The table below presents a preview of the EU MDR Technical Documentation Compliance Checklist and Guidelines:
Overview of the Gap Assessment Template: MDR Technical Documentation (EU MDR 2017/745)
Checklist Guide for Reference
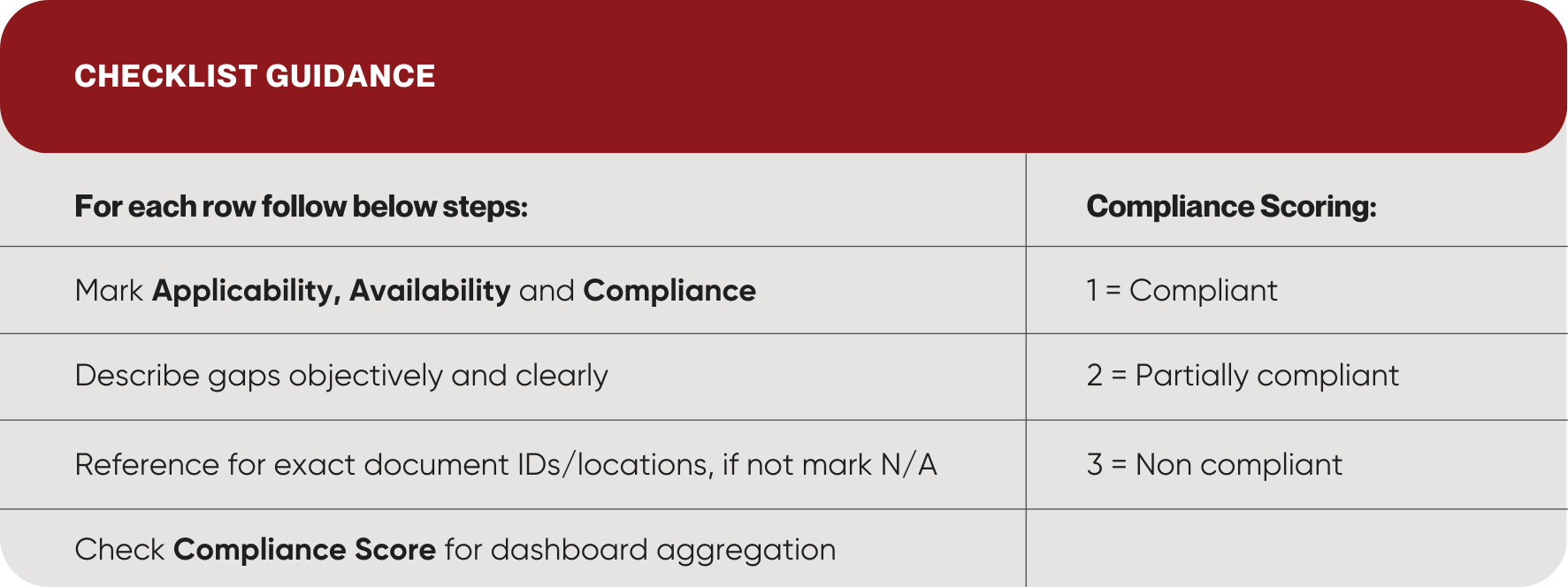
As inputs are entered by the medical device manufacturer into SJML’s exhaustive EU MDR Gap Assessment checklist, the dashboard will provide an overall evaluation of technical documentation efficiency and readiness through compliance scoring. The dashboard offers a consolidated view of conformity against Annex I (GSPR), Annex II, and Annex III, enabling an objective assessment of documentation completeness, traceability, and submission readiness.
Compliance status will be visualized by the MedTech manufacturer through a colour-coded scoring system:
- Green (Compliant): requirement fully addressed with objective evidence
- Amber (Partially Compliant): requirement addressed but requiring clarification, some incomplete or required updates
- Red (Non-Compliant): requirement not met or evidence missing, representing a potential Notified Body finding.
- Blue: Aggregate percentage score reflecting overall compliance status
Relevance of EU MDR Technical Documentation Compliance
(Regulation (EU) 2017/745 – Annex I, II & III)
SJML’s EU MDR Gap Assessment checklist supports a structured and systematic assessment of EU MDR technical documentation in alignment with Annex II and Annex III of Regulation (EU) 2017/745. It reflects current Notified Body expectations and incorporates recognized industry best practices.
This approach ensures:
- Each MDR requirement is evaluated for applicability and compliance
- Objective evidence is identified and referenced systematically
- Compliance gaps are identified early and addressed proactively
- Documentation updates are implemented efficiently, minimizing rework
Each applicable requirement is mapped to the relevant technical documentation section and supported by objective evidence. Cross-referencing to biocompatibility, clinical evaluation, risk management, PMS, and associated documents ensures full traceability and internal consistency.
The technical documentation demonstrates:
- Clear device description including intended purpose, users, patient population, variants, and accessories
- Controlled design and manufacturing processes supported by verification and validation activities
- Risk management aligned with ISO 14971 and supported by clinical evidence validating the benefit–risk profile within CER and PMS
- Clinical evaluation aligned with EU MDR requirements, MDCG guidance, and state-of-the-art practices
- Compliance with QMS requirements and risk-based biological safety evaluation aligned with ISO 10993-1
The documentation has been prepared for completeness, coherence, and proportionality in line with current Notified Body assessment expectations. Based on our research and analysis, it is considered ready for Notified Body conformity assessment, subject to closure of identified gaps.
We support manufacturers in developing Notified Body-ready technical documentation aligned with current EU MDR expectations. For support in closing compliance gaps and achieving full EU MDR Technical Documentation compliance for CE marking, or to access the complete 176-clause MDR compliance checklist and a detailed device-specific gap analysis, connect with our Regulatory Compliance experts.



