The functional performance of most modern medical devices depends on high reliability Printed Circuit Board Assemblies (PCBAs). PCBAs form the core of complex, life-critical devices ranging from patient monitors and infusion pumps to surgical robots. Therefore, they must fulfil stringent regulatory requirements and quality benchmarks.
The global medical electronics market is projected to reach $15.7 billion by 2029. Yet, a significant proportion of FDA medical device recalls stem from component and design issues, including electrical or PCB-related failures. This emphasizes that medical device PCBAs demand a regulatory compliance and quality-driven approach beyond high-precision manufacturing.
This article outlines a structured quality management approach for PCBA in complex medical devices. The proposed framework enables OEMs to align engineering, regulatory, and operational objectives from the outset.
1. Quality Management System (QMS) and Design Controls for PCBA
Quality management for PCBA begins in the design phase. A globally harmonized design process under ISO 13485:2016 ensures traceability, phase-gated reviews, and integrated risk and software lifecycle controls.
Best practices include –
- ISO 13485:2016 Compliance: Implement a risk-based quality management system aligned with ISO 13485:2016. It should include documented procedures for design inputs/outputs, design reviews, verification/validation, and design change management.
- Hardware–Software Risk Integration: Apply structured risk management per ISO 14971:2019 and software lifecycle control per IEC 62304. For devices with embedded software, ensure hazard analysis (e.g. FMEA/FTA) covers both hardware and software failure modes.
- Design Documentation: Maintain a comprehensive Design History File (DHF) and Device Master Record (DMR). Utilize electronic Quality Management Systems (eQMS) for traceability and change control.
- Regulatory Alignment: Ensure alignment with regional regulatory frameworks (e.g., US FDA 21 CFR 820, EU MDR/IVDR).
2. Medical PCBA Fabrication Standards and Safety Requirements
Medical PCBAs must meet IEC 60601-1 standards for electrical isolation, dielectric strength, and electromagnetic compatibility. This includes:
- Controlled creepage/clearance distances (per IPC-2221)
- Low EMI emissions and surge resistance
- Proper insulation of high-voltage circuits from patient-contact components
Material selection is critical. UL 94 V-0 flame-rated laminates and lead-free soldering are baseline. For high-speed digital or RF subsystems, stack-ups must be modeled for impedance and thermal gradients.
Non-compliance with IEC 60601-1 can significantly delay product timelines. According to MDDI, issues identified during third-party certification, including design flaws, often take weeks or months to resolve. These delays increase both time-to-market and overall costs.
3. Risk Management and Lifecycle Traceability: Ensuring Continuous Compliance
Under ISO 14971:2019, risk management is a continuous process that must evolve with clinical, technical, and regulatory realities. Mature organizations structure their Risk Management Files (RMFs) to:
- Map each identified hazard to the corresponding design control, risk mitigation strategy, and verification method
- Update dynamically based on post-market surveillance, clinical evidence, and CAPA outcomes
- Maintain regulatory readiness through version-controlled records linked to the DHF and DMR, with full authorship and timestamp trails
To operationalize this, leading firms implement traceability matrices within their eQMS that connect:
- Requirements → Design Inputs → Engineering Outputs
- Hazards → Mitigations → Verification → Validation
These matrices simplify technical dossier preparation, accelerate audit responses and mitigate inspection-driven design remediation.
4. Component-Level Traceability and Digital Quality Systems
Advanced traceability allows rapid containment, efficient root cause analysis and precise incident reporting to regulators. To implement unit-level traceability, leading MedTech organizations apply laser barcodes or QR codes to each PCB or subassembly. Each board is uniquely identified and linked to:
- Operator credentials, reflow oven temperature curves, and AOI inspection logs
- Component lot numbers, supplier batch IDs, and pick-and-place machine history
- Firmware versions, software configuration, and any subsequent rework or repairs
By linking serialization data with Enterprise Resource Planning (ERP) systems (e.g., SAP) and eQMS, firms achieve fully traceable Device History Records and effective batch control. This forms a crucial upstream input to global regulatory compliance requirements. According to McKinsey, leading MedTech OEMs that adopt digital quality backbones achieve up to 20% reduction in development time, enabling faster iterations and cost savings. These tools also promote simplicity and continuous improvement and allow organizations to adapt to changing regulations and markets.
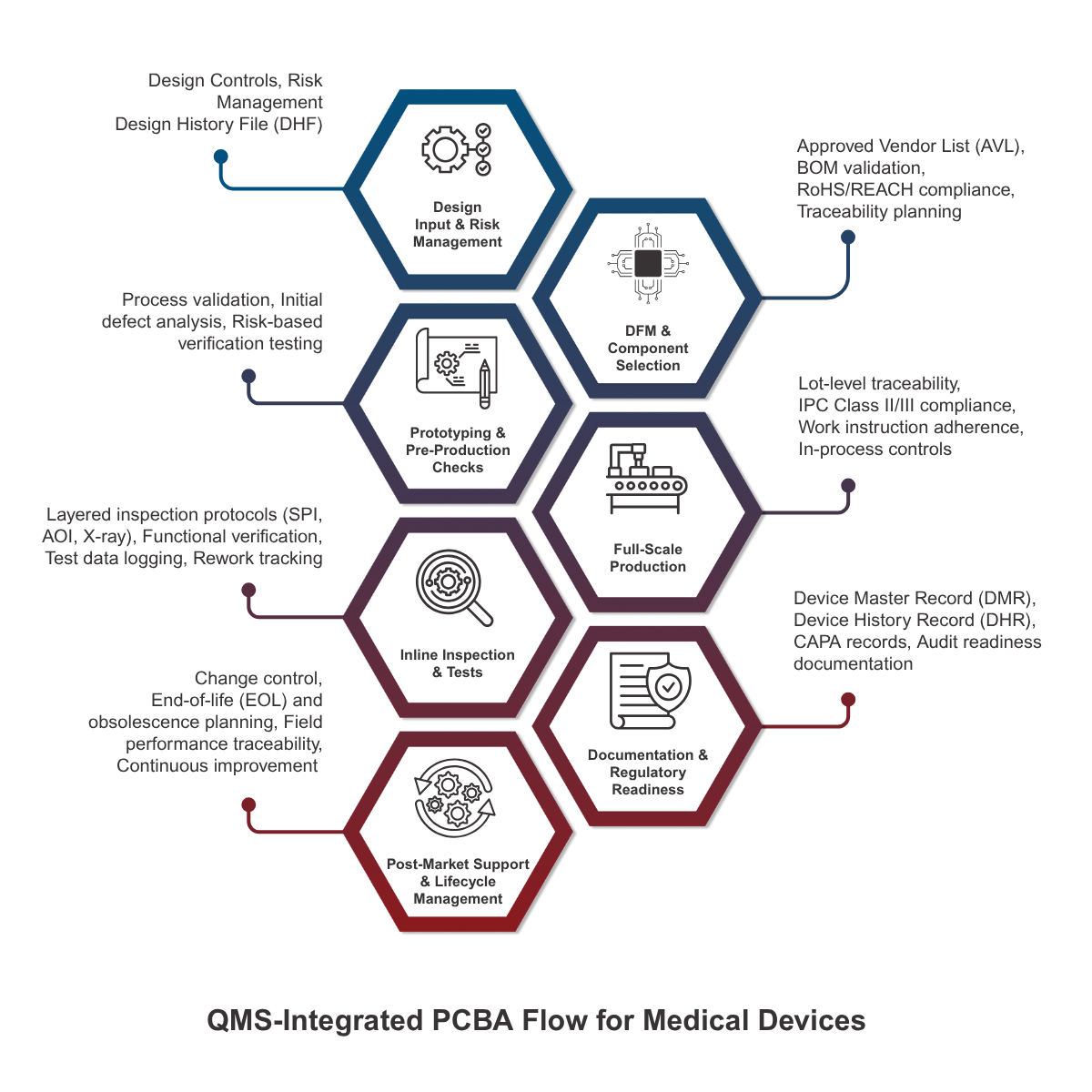
5. Design for Manufacturing, Reliability, and Sustenance
Class II and III medical devices require 10–15 years of product support, making sustainment planning essential at the Bill of Materials (BOM) level. Leading OEMs mitigate electronic obsolescence risk by:
- Selecting components with published lifecycle data and 5+ years of roadmap visibility (e.g., via IHS, SiliconExpert).
- Prioritizing components from multiple suppliers with confirmed RoHS/REACH certifications.
- Ensuring pin-compatible alternates for microcontrollers, op-amps, and critical sensors wherever feasible. This allows future drop-in replacements without needing board redesign or revalidation.
- Segmenting BOMs into A/B/C criticality tiers to develop proactive sourcing and requalification plans.
Seasoned organizations integrate Design for Manufacturing (DfM), Design for Assembly (DfA), Design for Test (DfT), and Design for Reliability (DfR) into early-stage design reviews. For example:
- DfM: Design for single-sided placement or reflow oven uniformity to reduce thermal stress
- DfT: Include built-in test points for in-circuit testing and boundary scan diagnostics
- DfR: Apply derating principles (IPC-9592) and validate thermal margins in environmental chambers
6. Global Regulatory Documentation and Submission Strategy
The following documentation elements are essential for regulatory-compliant medical PCBA manufacturing:
a. Technical File Requirements
Every component and critical process step in your PCBA must be backed by detailed technical documentation. This includes CAD files, PCB layout schematics, materials specifications, inspection criteria and test protocols.
b. Version Control
Medical device development is a living process, and your documentation must reflect that. Establish strict version control for Standard Operating Procedures (SOPs), design drawings, firmware revisions and software tools.
c. Validation Protocols
Manufacturers must demonstrate that both the equipment and processes used in PCBA production are robust and reproducible. This requires full Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ) documentation.
d. Regulatory Submission Readiness
PCBA documentation must be aligned with the submission requirements of target markets, whether it’s a 510(k) or PMA submission to the US FDA, a CE Mark technical file in the EU, or global dossiers for Canada, Japan, or Australia.
Case Study: Early Quality Planning vs. Late-Stage Hurdles in Medical Device PCBAs
| Aspect | Company A: Proactive Planning | Company B: Reactive Remediation |
| Approach to PCBA Documentation | Adopted documentation best practices from day one. | Treated documentation as an end-stage requirement. Relied on scattered records and inconsistent updates. |
| Version Control Implementation | Strict versioning of SOPs, CAD files, BOMs, and firmware via a QMS-integrated PLM system. | Manual tracking of revisions. Several undocumented firmware updates detected during pre-submission review. |
| Validation Readiness | Conducted full IQ, OQ, and PQ validations during pilot runs. Included process parameters in control plans. |
Skipped formal validation in early production. Requalification was required after FDA feedback.
|
| IEC 60601-1 Compliance Strategy | Built IEC 60601-1 compliance into board layout and design reviews. | Assumed compliance based on datasheet specs. Failed third-party safety testing and required PCB redesign. |
| Regulatory Submission Outcome | Received 510(k) clearance within 90 days due to complete documentation. | Submission delayed by 7 months. FDA issued AI request citing incomplete documentation and noncompliant test data. |
| Cost Impact | Higher upfront cost for process design, but minimal rework costs. | Incurred over $300,000 in redesign and remediation costs. |
| Time-to-Market | 13 months | 21 months due to rework and corrective actions. |
| Team Burden | Early cross-functional alignment. Minimal last-minute overheads. | Teams overwhelmed with rework. Loss of momentum. |
Key Takeaway
Proactive investment in PCBA quality and documentation may increase early-stage costs, but it prevents far greater downstream losses.
7. Comprehensive Inspection and Test Strategy for High-Reliability Medical PCBAs
A robust inspection and testing strategy is essential to ensure the reliability and safety of medical PCBAs. By combining advanced inspection techniques with thorough functional testing, manufacturers can identify defects early and guarantee consistent performance.
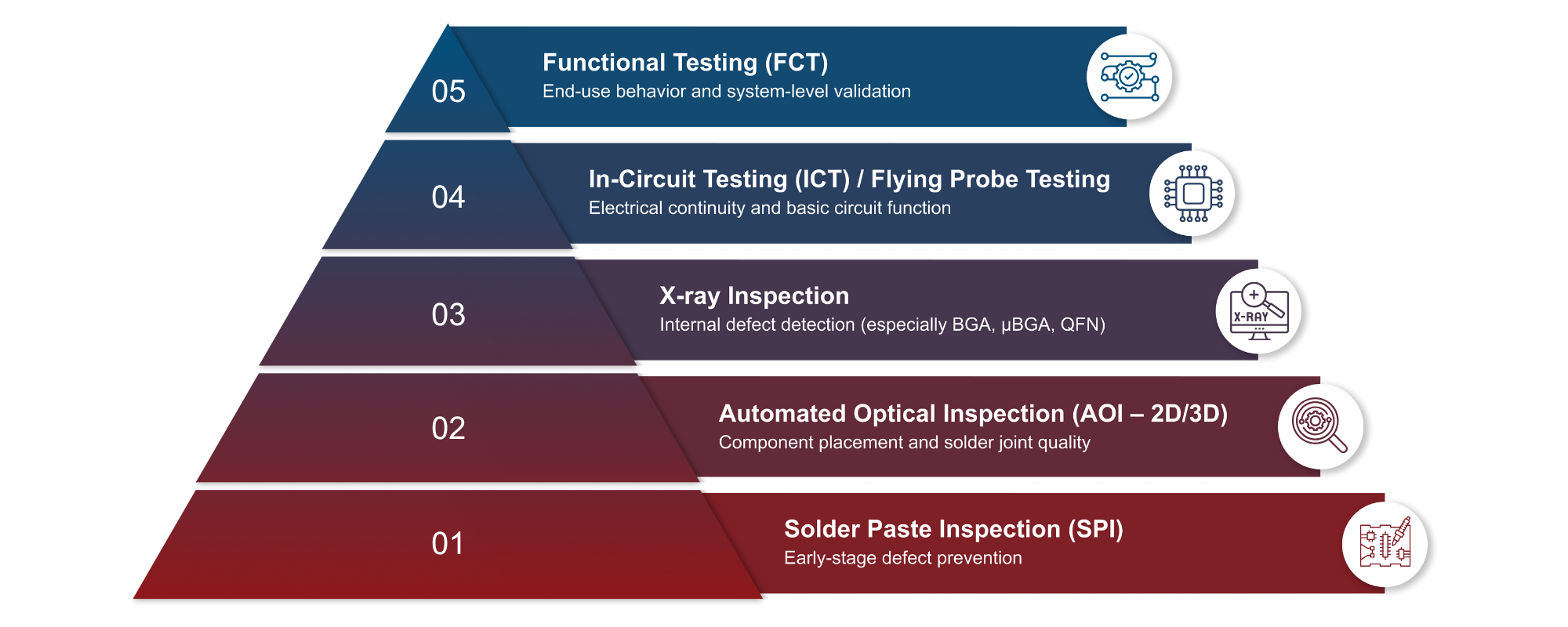
Syrma Johari MedTech’s advanced PCBA facilities support:
- Surface Mount Technology (SMT), Through-hole Technology (THT), and hybrid assembly lines
- Fine-pitch placement down to 12 mil and ultra-miniature components as small as 01005
- Support for rigid, flex, and rigid-flex PCBs up to 534 mm × 610 mm
- Automated conformal coating, potting, and encapsulation, with plasma surface treatment for high-reliability PCBAs
- N₂-enabled reflow ovens and BGA rework stations
- Laser marking for serialized PCB barcoding
Our Advanced Inspection Modalities
- Solder Paste Inspection (SPI): High-speed, non-contact inspection to detect solder deposition inconsistencies prior to component placement.
- 2D & 3D Automated Optical Inspection (AOI): Inline systems that verify component presence, orientation, polarity and placement accuracy using both surface and depth imaging.
- X-ray Inspection for BGA/μBGA Packages: Non-destructive imaging to assess hidden solder joints, identify voids, bridging and detect latent defects not visible through optical methods.
Comprehensive Testing Infrastructure
Our in-house testing systems are designed to ensure electrical integrity, design validation and long-term reliability:
- In-Circuit Testing (ICT): Validates electrical performance, including resistance, capacitance and analog signal accuracy in high-volume production.
- Flying Probe Testing: Ideal for prototypes and low-to-medium volumes, enabling flexible, fixture-less testing of nets and component behavior.
- Custom Functional Testing: Purpose-built test jigs replicate real-world use cases to assess power-on behavior, signal response, I/O performance and safety features.
These capabilities support regulatory compliance and design verification by generating objective manufacturing evidence. They also enable traceability, predictive maintenance insights and continuous process improvement across production batches.
8. Supplier Qualification, Risk Monitoring, and Obsolescence Control in Medical PCBAs
Component quality drives medical device reliability and compliance, and must be ensured through rigorous supplier monitoring.
Qualified suppliers are expected to meet ISO 13485:2016-compliant QMS criteria. Advanced OEMs maintain tiered Approved Vendor Lists (AVLs), audited at defined intervals for:
- Supplier change notification protocols
- Material composition compliance (RoHS/REACH)
- CAPA handling efficiency and batch-level documentation integrity
Furthermore, they apply Design for Sustenance frameworks outlined earlier to prevent manufacturing and regulatory compliance disruptions caused by obsolescence.
9. Environmental and Chemical Compliance: RoHS and REACH Alignment
Compliance with environmental and chemical regulations is a critical aspect of medical PCBA manufacturing. It ensures product safety, supports regulatory approval, and enables sustained market access.
a. RoHS 3 (EU Directive 2015/863):
This directive restricts ten hazardous substances, including lead, mercury, cadmium, hexavalent chromium, and four phthalates. Manufacturers must demonstrate compliance through supplier declarations. This is validated by analytical methods such as X-ray fluorescence (XRF) spectrometry and verified during incoming inspections. It is important to note that medical devices may have specific exemptions, which require careful review and monitoring for updates.
b. REACH SVHC Control:
Every BOM line item must be screened for Substances of Very High Concern (SVHCs) according to the European Chemicals Agency (ECHA) guidelines. OEMs are responsible for maintaining accurate material declarations and supplier Material Safety Data Sheets (MSDS) and documenting substitution plans where applicable. Additionally, safe handling and disposal instructions must be included in SOPs.
c. Process Compliance and Validation:
Use of halogen-free fluxes, lead-free solders and conformal coatings that meet Volatile Organic Compound (VOC) and outgassing limits is mandatory. Validation techniques such as ionic contamination testing and Surface Insulation Resistance (SIR) analysis ensure process compliance. Additionally, supplier audits and quarterly material risk reviews reinforce ongoing compliance.
d. Supply Chain Communication:
Effective compliance requires cascading these requirements down the entire supply chain. Tiered suppliers must be qualified to meet RoHS and REACH standards. All compliance documentation must be collected and verified to maintain traceability and minimize risk.
e. Record Retention and Audit Preparedness:
All compliance evidence, including certificates of analysis, laboratory test reports and supplier declarations, should be systematically archived within the eQMS.
10. Quality-Driven PCBA Partnerships for Long-Term Market Success
OEMs scaling medical electronics need PCBA partners who can:
- Ensure full component and process traceability
- Validate complex assemblies with tailored test strategies
- Support long product lifecycles with ongoing compliance
- Operate in strict alignment with ISO 13485:2016 and global MedTech standards
At Syrma Johari MedTech, we offer high-precision medical PCBA services with uncompromising quality and regulatory rigor. Our advanced capabilities combine manufacturing excellence and global regulatory compliance, enabling efficient scale-up and sustained market success.

