In vitro diagnostic (IVD) tests are essential medical tools to diagnose and monitor a wide range of diseases. Among the various IVD techniques, immunoassays are one of the most widely adopted bioanalytical platforms. They detect and quantify biomarkers by utilizing the antigen–antibody binding mechanism.
Immunoassay Market Insights
Over time, immunoassays have evolved into multiple formats, each with distinct clinical applications and detection techniques. Common types include fluorescence immunoassay (FIA), enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, colorimetric immunoassay, and chemiluminescence immunoassay (CLIA).
The global immunoassay market is projected to grow from USD 35.53 billion in 2025 to USD 47.76 billion by 2030. Within the global immunoassay market, the FIA segment is expected to grow at ~5% CAGR in the next decade as per internal analysis.
The growing adoption of FIA-based systems is opening the door for strategic collaborations and next-generation testing technologies. Here is a look at the 8 breakthrough technology trends in FIA shaping the future of next-generation in vitro diagnostics (IVDs) in 2025. More than half of the laboratories worldwide today prefer FIA over conventional methods due to its high sensitivity and specificity.
Emerging Trends in Fluorescent Immunoassays (FIA) Technology
1. Growing Adoption of Automation and AI in Diagnostic Laboratories
Embracing automation and artificial intelligence (AI) in FIA platforms is now considered essential for efficient, high-throughput testing.
- Advanced lab automation systems have streamlined pre-analytical and analytical assay workflows.
- Machine learning algorithms can assist in interpreting fluorescent signals on immunoassay strips, improving result accuracy in real time.
- AI-driven reflex testing and result validation is anticipated to shorten diagnostic workflows and maintain quality. This is crucial as labs combat personnel shortages and rising sample volumes.
Regulatory Perspective
Regulatory bodies are working to harmonize frameworks that address the unique complexities of AI in medical and diagnostic devices.
- In early 2025, the International Medical Device Regulators Forum (IMDRF) released Good Machine Learning Practice (GMLP) for Medical Device Development. This document outlines a unified set of principles for the development of safe and effective AI/ML-enabled medical devices.
- In the United States, the FDA regulates AI-enabled medical devices and IVDs primarily through established pathways, including the 510(k), De Novo, and Premarket Approval (PMA) processes.
- AI-enabled IVDs are subject to two regulatory frameworks in the European Union: In Vitro Diagnostic Regulation (IVDR) and the EU AI Act, 2024.
2. Rapid Expansion of IoMT and Connected FIA Devices
The Internet of Medical Things (IoMT) is making in-roads in diagnostics. Integrated, automated machines will enhance machine-to-machine (M2M) communication between instruments, robots, and “smart” consumables in the lab.
Connected FIA systems continuously monitor performance and maintenance needs, enabling a more sustainable and adaptive lab environment.
- New-generation FIA analyzers like bioMérieux’s VIDAS 3 are bidirectionally connected to laboratory information systems (LIS) and feature remote access for troubleshooting.
- Vision-guided systems using LiDAR and deep learning allow robots to navigate lab environments and manage samples or reagents without collisions.
- The QuidelOrtho Sofia 2 is a point-of-care (POC) analyzer for flu and COVID-19 that can wirelessly transmit data to cloud for real-time epidemiological tracking.
Cybersecurity Regulations
With increasing connectivity in FIA and other medical devices, the FDA has strengthened its cybersecurity guidance (2025) to safeguard patient data and ensure safety. Manufacturers must integrate cybersecurity measures throughout the lifecycle of a “cyber” medical device. This includes:
- Adopting a Secure Product Development Framework (SPDF) to identify and mitigate risks
- Submitting specific documentation during the premarket review process
- Maintaining robust post-market surveillance programs
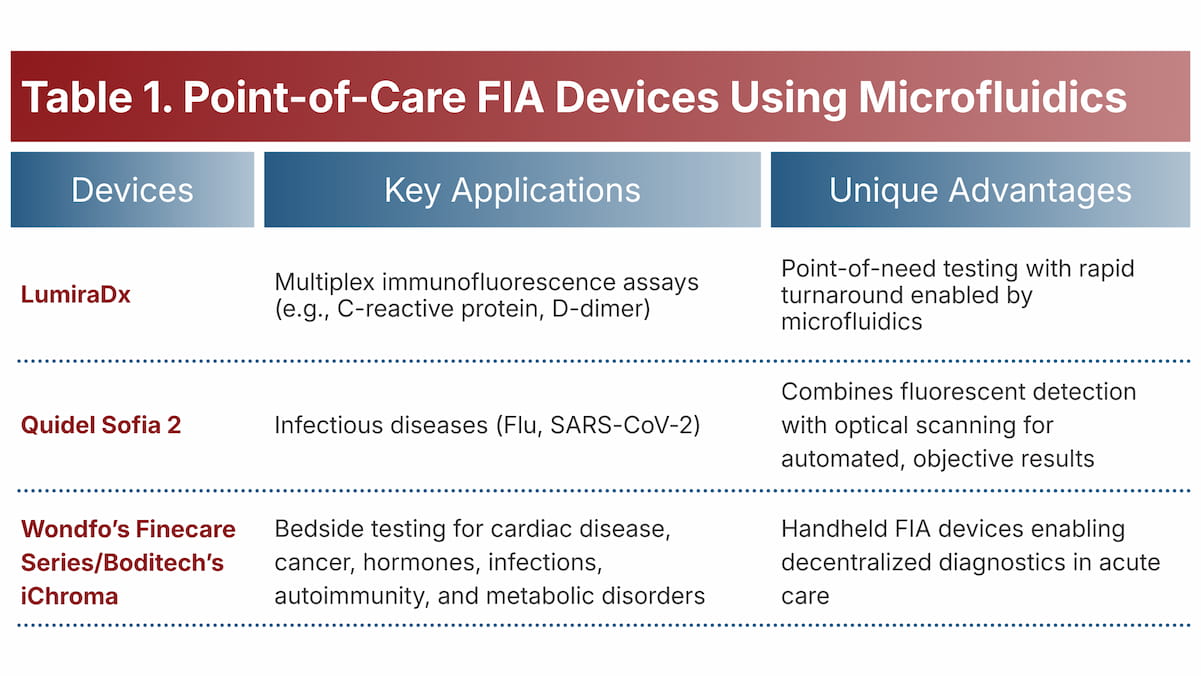
3. Integration of Microfluidics in Point-of-Care FIA Platforms
Breakthroughs in microfluidics and lab-on-a-chip technologies are powering smaller and faster point-of-care (POC) fluorescent analyzers.
The global microfluidics market, estimated at USD 22.43 billion in 2025, is projected to reach USD 32.67 billion by 2029. This expansion is driven by the rising adoption of microfluidics in POC diagnostic devices, in addition to broader applications.
Disposable microfluidic cartridges integrated with nanoparticle labels and AI-based image analysis can deliver lab-quality precision at a fraction of the cost. A deep learning-enhanced vertical flow assay on a paper-based chip quantified cardiac troponin I (a heart attack marker) in just 15 minutes with high sensitivity.
Commercial platforms are scaling these innovations to decentralize diagnostics, while preserving the sensitivity and reliability of fluorescence (Table 1).
4. Emergence of Ultra-Sensitive Digital Immunoassays
Ultra-sensitive immunoassays are breaking new ground by detecting biomarkers once measurable only with invasive or complex methods.
- Quanterix’s SiMoA (Single Molecule Array) platform uses microbead arrays and fluorescent readouts to count individual immune complexes at femtogram levels. This sensitivity is far beyond the detection limits of conventional immunoassays.
- Additionally, the Quanterix plasma p-Tau 217 assay received FDA Breakthrough Device designation in 2024 for its potential to transform early Alzheimer’s diagnosis.
5. Rising Demand for Multiplex and Panel-Based FIA Systems
Panel-based systems improve throughput and can reveal biomarker patterns often missed by single-analyte tests, delivering a broader clinical picture.
- A notable breakthrough is the Penta-Plex Alzheimer’s Disease Capture Sandwich Immunoassay (5ADCSI), a low-cost blood test that detects five biomarkers at once. It relies on the Luminex xMAP fluorescent bead technology and has strong potential of accessible, early diagnosis and intervention.
- Multiplex FIA platforms are being applied in infectious diseases, neurology and cytokine profiling for immune monitoring.
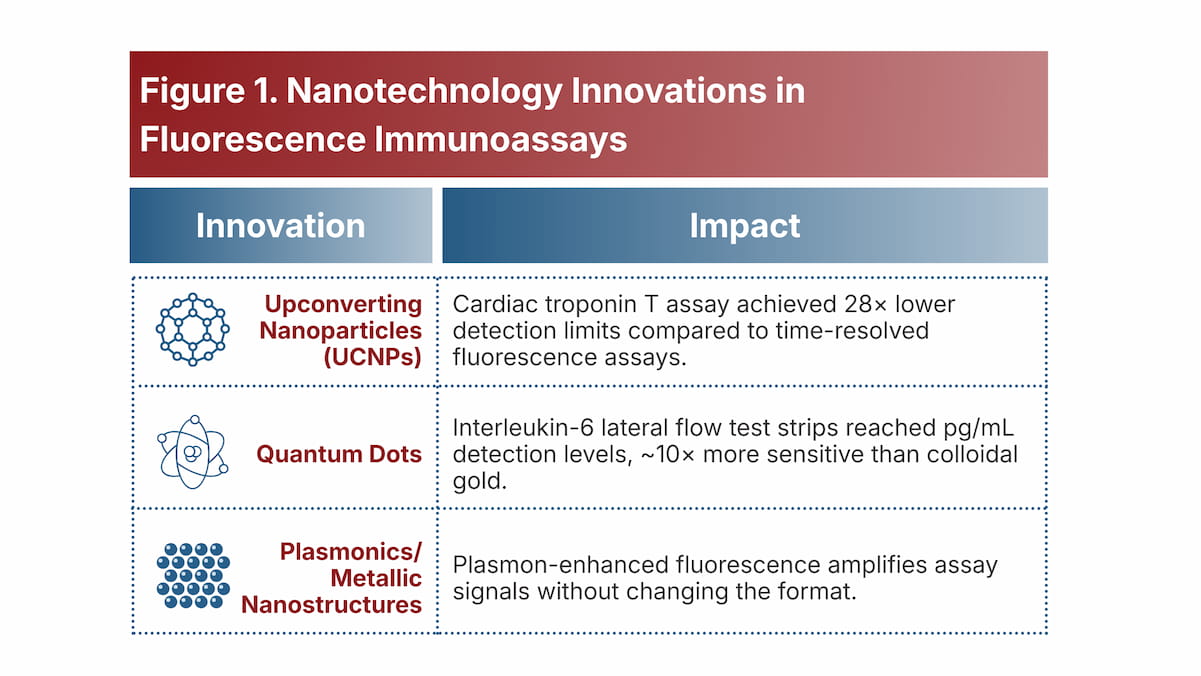
6. Increasing Use of Nanotechnology and Advanced Fluorescent Labels
Modern immunoassays are exploiting nanotechnology to break sensitivity barriers, enabling earlier disease detection. Novel reporters like upconverting nanoparticles (UCNPs) and quantum dots are used as labels in FIA due to their bright, stable signals and low background (Figure 1).
7. Shift Toward High-Throughput FIA Testing
Today’s immunofluorescent analyzers are closing the gap in terms of both, speed, and precision. Over 55% of new FIA devices feature advanced automation to boost throughput and efficiency.
- A prime example is the Tosoh AIA-2000, a fully automated, fluorescent enzyme immunoassay (FEIA) analyzer which processes up to 200 tests/hour across a wide assay menu.
- The Wondfo Finecare X2, a 16-channel FIA analyzer built for small-to-medium labs, also achieves ~200 tests/hour. It supports a broad test panel, from cardiac and inflammatory markers to hormones, diabetes, and tumor diagnosis.
8. Implementation of Smart FIA Ecosystems
Beyond connectivity, fluorescence immunoassay systems are integrating with cloud platforms for real-time data analytics.
- Networked FIA analyzers not only process tests but also self-monitor for quality, flag instrument drift, and share results in real time.
- At scale, aggregated FIA datasets are already being mined for biomarker signatures in diseases like Alzheimer’s and dementia.
- Early prototypes have explored voice-controlled AI assistants for lab staff.
While these advancements are nascent, they signal a shift from standalone instruments to smart, connected diagnostics ecosystems.
Syrma Johari MedTech – Your Trusted Partner for IVD Design and Manufacturing
A leading contract design and manufacturing partner, Syrma Johari MedTech brings 45+ years of proven for expertise spanning microfluidic cartridge design, injection molding for IVD devices, automated bonding, and PCB assembly for diagnostic platforms. Our team of IVD domain experts have hands-on experience in development of multiplex FIA systems, point-of-care analyzers, and cutting-edge diagnostic devices.
From concept to aftermarket, our end-to-end IVD manufacturing solutions ensure regulatory compliance and speed-to-market. That is why leading MedTech innovators choose us as their most trusted IVD design and manufacturing partner.

